Production of copper-doped calcium phosphate and its evaluation of deodorizing and antibacterial properties
- What is calcium phosphate?
- Deodorant properties of calcium phosphate
- Copper-containing hydroxyapatite(Cu-HA)
- Addition to calcium phosphate
- Purpose of research
- Slides
Calcium phosphate is a solid contains phosphate ions [PO4-] and calcium ions [Ca2+], and is found in abundance in nature as a component of apatite and the bones of living organisms. Hydroxyapatite is a particularly well-known example. Hydroxyapatite is the most familiar form of calcium phosphate, accounting for 60% of human bones and 97% of tooth enamel.
The type of calcium phosphate is determined by the ratio of Ca and P in the crystal (Fig. 1). Since calcium phosphate has different characteristics depending on the type, it has a wide range of applications (Fig. 2).

Fig.1. Types of calcium phosphate and the ratio of Ca and P

Fig.2. Applications of calcium phosphate[1]
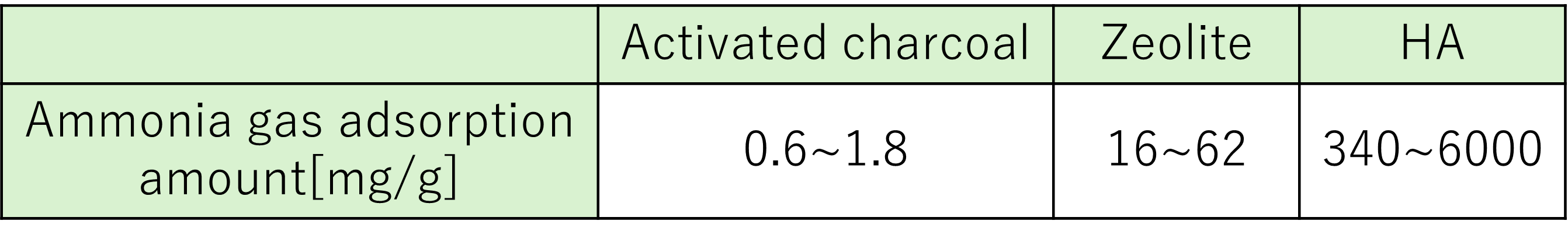
Calcium phosphate has a deodorizing effect, and research has shown that hydroxyapatite (hereinafter, "HA") has an ammonia adsorption rate per mass that is several hundred to several thousand times that of activated carbon and zeolite (Table 1).
Table 1. Ammonia gas adsorption amount of various deodorants (unit: mg/g)[2]
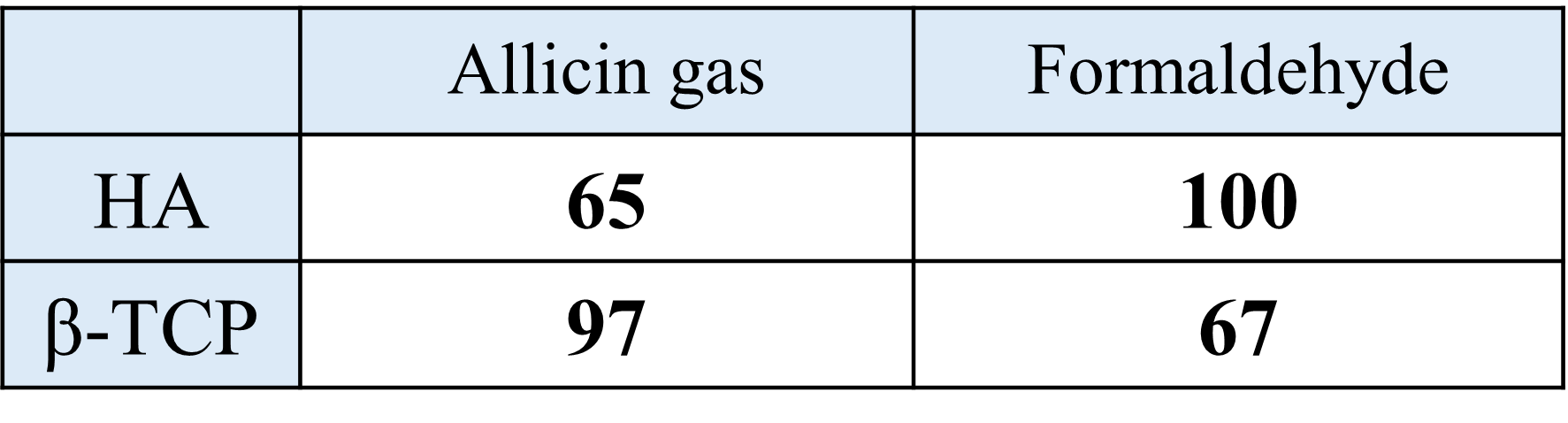
Previous research has revealed that different types of calcium phosphate have selectivity for deodorizing gases (Table 2).
Table 2. Deodorizing gas selectivity of hydroxyapatite and β-TCP (unit: %)[3]
Antibacterial effect and deodorant effect by heavy metal
Heavy metal ions are known to have antibacterial effects. It is said that antibacterial properties are achieved when ionized heavy metals are taken up by bacteria, but the antibacterial mechanism behind this has not been elucidated. Several theories have been proposed, including the inhibition of protein production by heavy metal ions and the oxidative effect of active oxygen.
There are various types of heavy metals that exhibit antibacterial properties (Fig. 3). Many of these metals are expensive precious metals or are known to have adverse effects on the human body, and safe antibacterial metals are limited to Ag, Cu, etc.
Heavy metals can be used as deodorants by using their antibacterial effects to kill odor-causing bacteria.
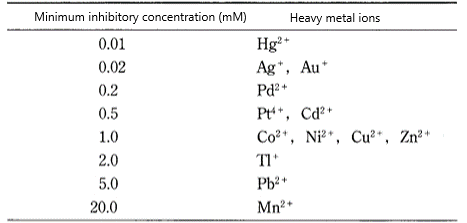
Fig.3. Antibacterial properties of heavy metal ions[3]
Due to the ion exchange properties of calcium phosphate, it is possible to incorporate metal ions into the crystals of calcium phosphate. As a result, it is possible to combine the deodorant effect of calcium phosphate with the deodorant effect of antibacterial properties, and it is possible to create a more effective deodorant. Cu salt was dissolved in Ca(OH)2 suspension or H3PO4 aqueous solution. There is a solution method that reacts Ca ions, P ions, and Cu ions in a solution by mixing.
In this study, we aim to establish a method for producing Cu-containing calcium phosphate, and by verifying the compatibility of high effectiveness and safety through antibacterial and cytotoxicity evaluations, we will explore new materials that can be used to make better deodorizing products.
[1](Left)Tsuno group Ltd. HP
https://www.tsuno.co.jp/products/fertilizer-feed/dibasic-calcium-phosphate/"
[1](Center)Olympus Biomaterial Ltd. HP
https://www.biomaterial.co.jp/jp/news/2006/nr002.html
[1](Right)Dr.apatite Ltd. HP
https://drapatite.com/?yclid=YSS.EAIaIQobChMImNKJjq7T_wIVAbKWCh0XLAV2EAAYASAAEgJcI_D_BwE
[2]Kazuhide, O.: KAKEN Database, "Deodorant function of hydroxyapatite thin film using sputtering technology" (2004)
[3]Yoshinobu, M.: Antibacterial properties of silver and copper ions Chemistry and education 53(5) (2005)より
